StageBio delivers integrated IHC, ISH, and immunofluorescence services underpinned by one of the industry's deepest portfolios of validated tissue biomarkers. Our pathology-led approach ensures data is biologically meaningful, reproducible, and trusted by regulators, giving you confidence that biomarker results truly reflect biology, not artifacts, which is critical for IND packages and translational research.
With 300+ validated antibody biomarkers spanning oncology, neuroscience, inflammation, fibrosis, vascular biology, and multiple species, we help clients accelerate study setup, reduce risk, and generate confident translational data.
StageBio's library of validated tissue biomarkers allows you to bypass lengthy antibody sourcing, optimization, and validation, accelerating study timelines and reducing risk. Our proven performance across IHC and IF means we know which markers to use, how to deploy them, and how to interpret the results with biological precision. Whether you need biomarker panels tailored to your therapeutic area or support for custom antibody development, we deliver clear mechanism of action tracking and immune response profiling that strengthens efficacy narratives and de-risks clinical transition. With pathologist-led expertise and regulator-ready data, we help biotech teams generate meaningful histopathology insights without delays.
Validated
Biomarkers
Species
Coverage
Targets for Tailored
Multiplex IF Panels
covering oncology, neuroscience, inflammation, and various diseases and species
delivering validated insights, regulatory-grade interpretation, and credibility you need to drive programs forward
StageBio offers a comprehensive suite of tissue-based biomarker services designed to accelerate drug development with regulatory-ready data. Our capabilities span immunohistochemistry (IHC), antibody and biomarker validation, in situ hybridization (ISH), and multiplex immunofluorescence (IF), supported by 300+ validated biomarkers and pathologist-led interpretation. Whether you need to confirm target engagement, validate novel assays, visualize gene expression, or map complex spatial biology, we deliver the translational insights that strengthen your program and de-risk clinical transition.
Antibody-based staining to detect and localize specific protein biomarkers directly within tissue sections, revealing where, how strongly, and in which cells a target is expressed.
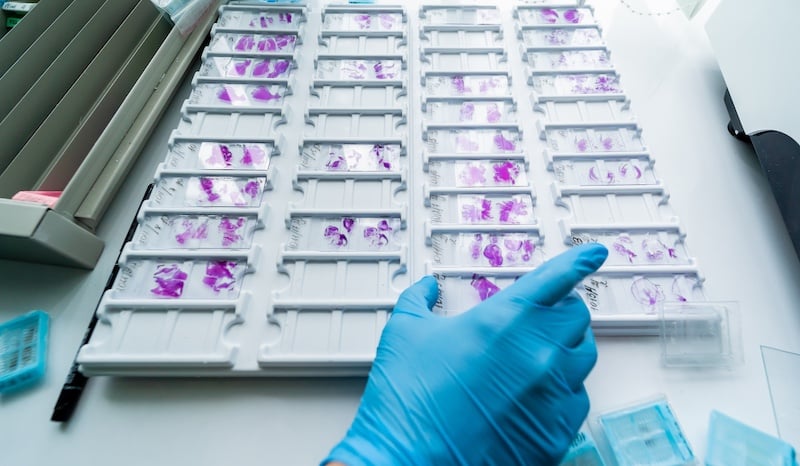
Our services deliver reliable biomarker data that supports mechanism-of-action studies, safety interpretation, and regulatory submissions. By providing mechanistic confidence beyond H&E, we help you build a stronger efficacy and differentiation story while increasing confidence as you move into the clinic.
Our Capabilities:
Confirms target engagement and mechanism of action
Enables cell-type identification and immune profiling
Advanced IHC and multiplex IHC to confirm target expression and localization
Screening and comparison of antibody clones to assess specificity and off-target binding
Quantitative digital pathology for scalable, reproducible tissue analysis
GLP-compliant toxicologic pathology to support IND submissions
Board-certified pathologist review with proven regulatory success
Our approach gives you confidence that biomarker results truly reflect biology, not artifacts, which is critical for IND packages and translational research.
Our Capabilities:
Optimization of dilution, antigen retrieval, and staining conditions
Cross-reactivity testing across species and strains
GLP-grade validation for regulated studies
Accelerated method development for novel or first-in-class targets
Deep expertise reduces unexpected safety or efficacy issues later in clinical development
Ensures translational continuity from preclinical to clinical stages
Rigorous testing, optimization, and verification of antibodies and biomarker assays prior to study deployment, ensuring accuracy, specificity, and reproducibility.
Detection of RNA or DNA sequences directly within tissue using sensitive platforms such as RNAscope, confirming gene expression and transgene localization when protein detection is unreliable or antibodies are unavailable.
Our assays add molecular-level context directly in tissue, strengthening mechanistic insight and generating publication-quality data. With higher specificity than many protein-based assays, we provide powerful translational and biomarker evidence that helps de-risk novel targets.
Our Capabilities:
Custom ISH method development tailored to target, tissue, and study goals
Expertise across RNAscope, Basescope, DNAscope, and chromogenic/fluorescent ISH
Control selection, probe validation, and tissue optimization
Integration with IHC and digital pathology for multimodal analysis
GLP-compliant ISH development for submission-quality studies
Higher specificity than many protein-based assays with reduced background
Our technology enables complex biology to be visualized clearly, making it ideal for oncology, CNS, immune profiling, and precision medicine programs. This provides rich, spatial biology insight that supports high-impact figures and publications while enhancing translational relevance.
Our Capabilities:
Tailored multiplex IF panels (2-8+ targets)
Direct, indirect, and TSA-based amplification strategies
High-resolution imaging with quantitative spatial analysis
Co-localization and spatial pathway analysis
Deep immune phenotyping and target modulation assessment
Publication-quality figures and high-impact datasets
Fluorescent antibody staining to visualize multiple biomarkers simultaneously within the same tissue section, revealing spatial organization and interaction of cells, targets, and pathways.
Connect with our team to explore how StageBio's advanced IHC and multiplex IF capabilities and board-certified pathologist reviews can de-risk your study, strengthen your data package, and support regulatory success.
StageBio Antibody Biomarker List
How Immunohistochemistry and Immunofluorescence Benefit Pre-Clinical and Clinical Research
IHC or IF: Which is Best for My Study?
An Overview of IHC Staining Procedures for Formalin-Fixed, Paraffin-Embedded (FFPE) Tissues
I have evaluated thousands of slides that StageBio has prepared. Their consistency from slide to slide is remarkable. It makes my job as a pathologist much easier when we contract our work to StageBio! Director of Pathology, Contract Research Organization
We've always found the quality of work and GLP reporting we receive from StageBio to be top-notch. Good customer service is hard to come by, but that is one of the things that keeps us coming back. Assistant Director of Medical Devices, Medical Device CRO