With 20+ years of pathology expertise and regulatory insight, we help you navigate the pitfalls that often derail medical device development: incomplete planning, inadequate sample collection or processing, need for advanced techniques and specialized stains that compromises data quality, and the costly assumption that all suppliers offer equivalent pathology services.
We have conducted more than 1,000 Non-GLP and GLP medical device pathology studies across multiple therapeutic areas, delivering the specialized expertise and proactive guidance to move programs forward from day one.
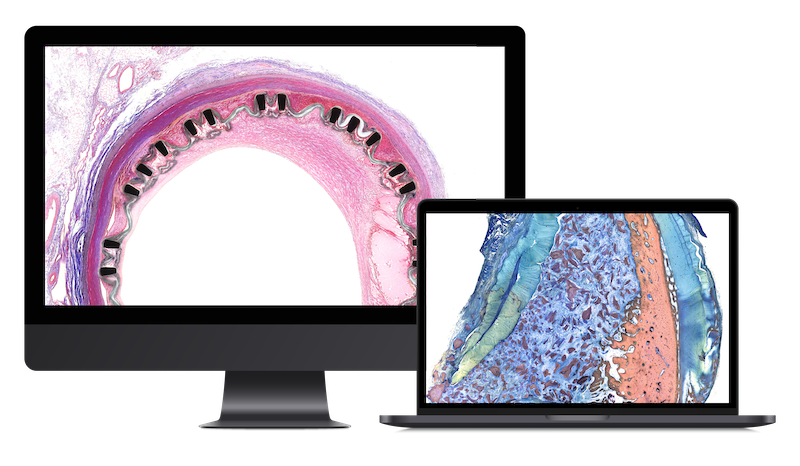
Cardiovascular Device Studies
Nervous System Device Studies
Musculoskeletal & Dental Device Studies
bringing breadth and depth, accelerating turnaround times and strengthening regulatory confidence
delivering validated insights, regulatory-grade interpretation, and credibility you need to drive programs forward
We offer a range of specialized GLP-compliant pathology services for medical devices to support your project from preclinical studies through regulatory submission. With our proven experience and deep expertise, we deliver the quality, timelines, and regulatory assurance you need, while providing strategic insights that add true value to your program.
Download our flyer for a full list of services we offer for medical device evaluation.

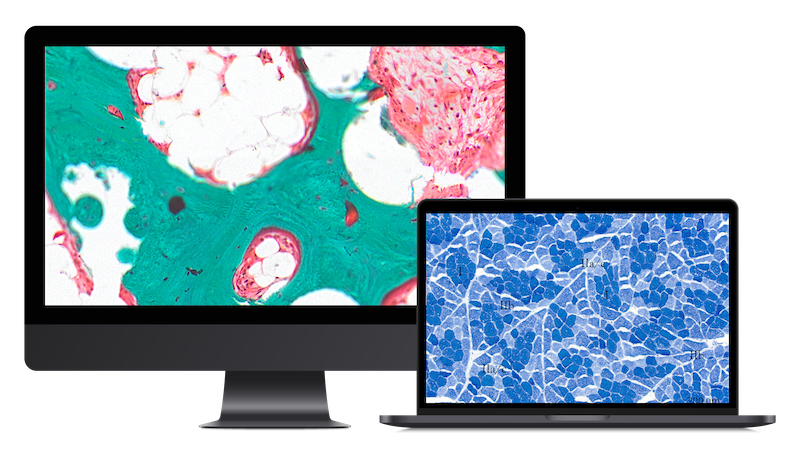
We conduct robust non-clinical evaluations (necropsy, histopathology, morphometry, image analysis) of devices and biomaterials in any tissue but specially in the following areas:
We offer a wide platform including bespoke histopathology techniques, imaging, and analytics for:
Our quality-driven scientific team is dedicated to open collaboration and flexible support. StageBio provides a tailored approach with clear, insightful interpretation to support you with:
Connect with us for an initial discussion or request for proposal.
Receive expert advice on your scope of work from our principal investigator, a pathologist, and other specialists.
Collaborate with our team to develop the histopathology strategy specific to your objectives.
Receive our StageBio estimate, undergo scheduling and launch the project.
Director of Pathology
Contract Research Organization
Study Director
Medical Device Organization
Jaime Paulin, DVM, MS, DACVP
StageBio, Senior Pathologist