Immunohistochemistry (IHC) and immunofluorescence (IF) are molecular assays that involve the use of antibodies to detect specific proteins within tissues on microscope slides
With IHC, the proteins are visualized with a colored chromogen and viewed with a brightfield microscope. Whereas with IF, the proteins are visualized with a fluorochrome and viewed with a fluorescence microscope. Each of these methods can be used on either formalin-fixed, paraffin-embedded (FFPE) tissue or snap-frozen, cryosectioned tissue. IHC and IF can be used to detect a single protein in tissues or antibodies can be multiplexed to detect two or more proteins on one slide. Multiplexing is an excellent way to view how proteins are interacting together within the tissue or to view several different cell populations at once. Each of these methods has advantages and disadvantages.
Chromogenic IHC generally involves using a DAB chromogen with a hematoxylin counterstain. The most common form of DAB is brown; this produces slides with crisp brown positive staining with blue nuclei. Recently, DAB has become available in additional colors such as purple, red, green, yellow, and even black. Alternate counterstains, such as Methyl Green or Nuclear Fast Red can be used to highlight the positive staining with these colored chromogens. One of the biggest advantages of IHC over IF is the fact that IHC staining is permanent and does not fade like IF staining does. The slides can be archived and viewed years, even decades, later and the staining should still look the same. In addition, tissue architecture and morphology are more readily apparent in IHC stained slides, making interpretation easier.
Multiplexing chromogenic IHC is commonly used for highlighting different cell populations that do not overlap. However, this is not the best choice, if co-localization within a cell is desired. Below are some photos of GFAP chromogenic IHC stains with different colored chromogens:
%20Hem%20counterstain_square.jpg?width=941&name=GFAP%20(brown)%20Hem%20counterstain_square.jpg) |
%20Hem%20counterstain_square.jpg?width=941&name=GFAP%20(green)%20Hem%20counterstain_square.jpg) |
|
GFAP with traditional DAB (brown) Chromogen and Hematoxylin (blue) counterstain |
GFAP with Green Chromogen and Hematoxylin (blue) counterstain |
%20Green%20counterstain_square.jpg?width=941&name=GFAP%20(purple)%20Green%20counterstain_square.jpg) |
%20Hem%20counterstain_square.jpg?width=941&name=GFAP%20(red)%20Hem%20counterstain_square.jpg) |
|
GFAP with Purple Chromogen and Methyl Green counterstain |
GFAP with Red Chromogen and Hematoxylin (blue) counterstain |
IF is the staining method of choice for multiplexing, particularly when co-localization is desired or when more than 2 different antibodies are needed. With IF staining, antibodies against specific proteins are added to the slide and are then visualized with fluorescent dyes and viewed with a fluorescent microscope.
Below is a list of fluorochromes that are commonly used at HSRL. HSRL has a Hamamatsu S60 scanner which can IF stained slides.
- FITC (fluorescein)/Cy2/Alexa Fluor 488 (green)
- Texas Red/Alexa Fluor 594 (red)
- Cy5/Alexa Fluor 647 (far red)
- Rhodamine/Cy3/TRITC/Alexa Fluor555 (greenish-yellow)
- DAPI/Hoechst (blue)
Multiplex IF staining is an effective method to identify distinct cell populations as well as those that express more than one marker. It is becoming very common in immuno-oncology research, in which multiple different cell populations can be viewed on one slide.
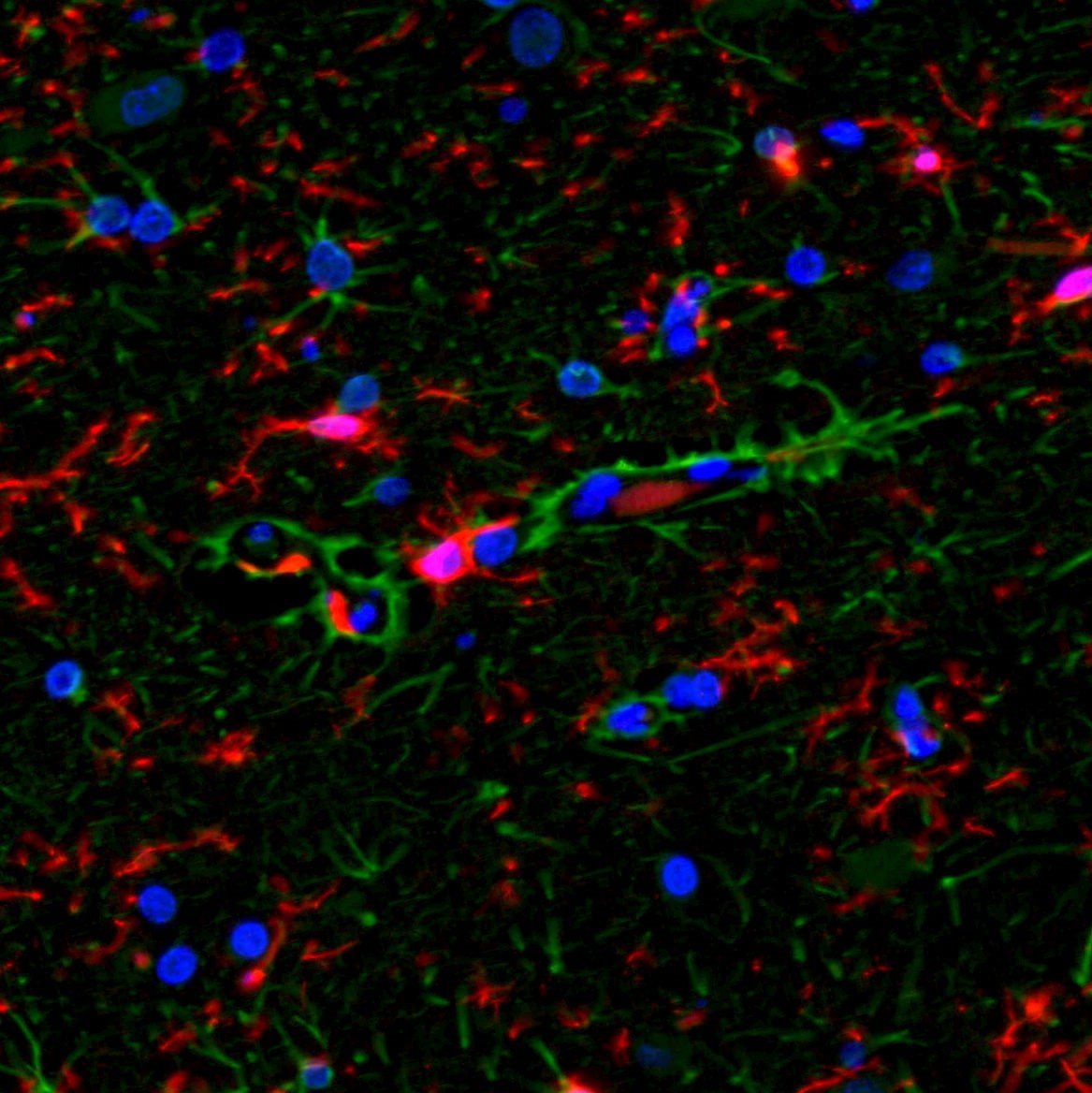
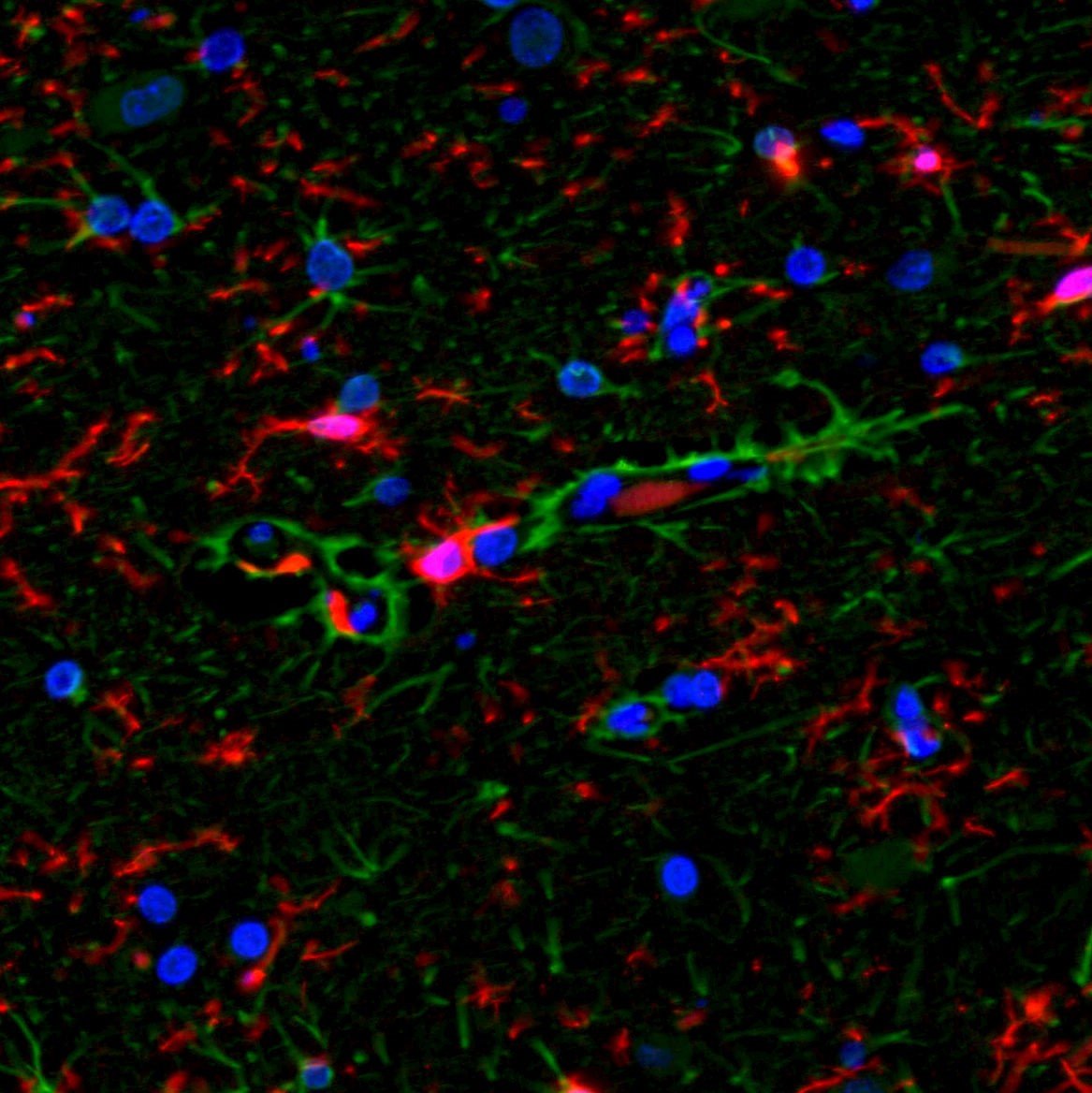
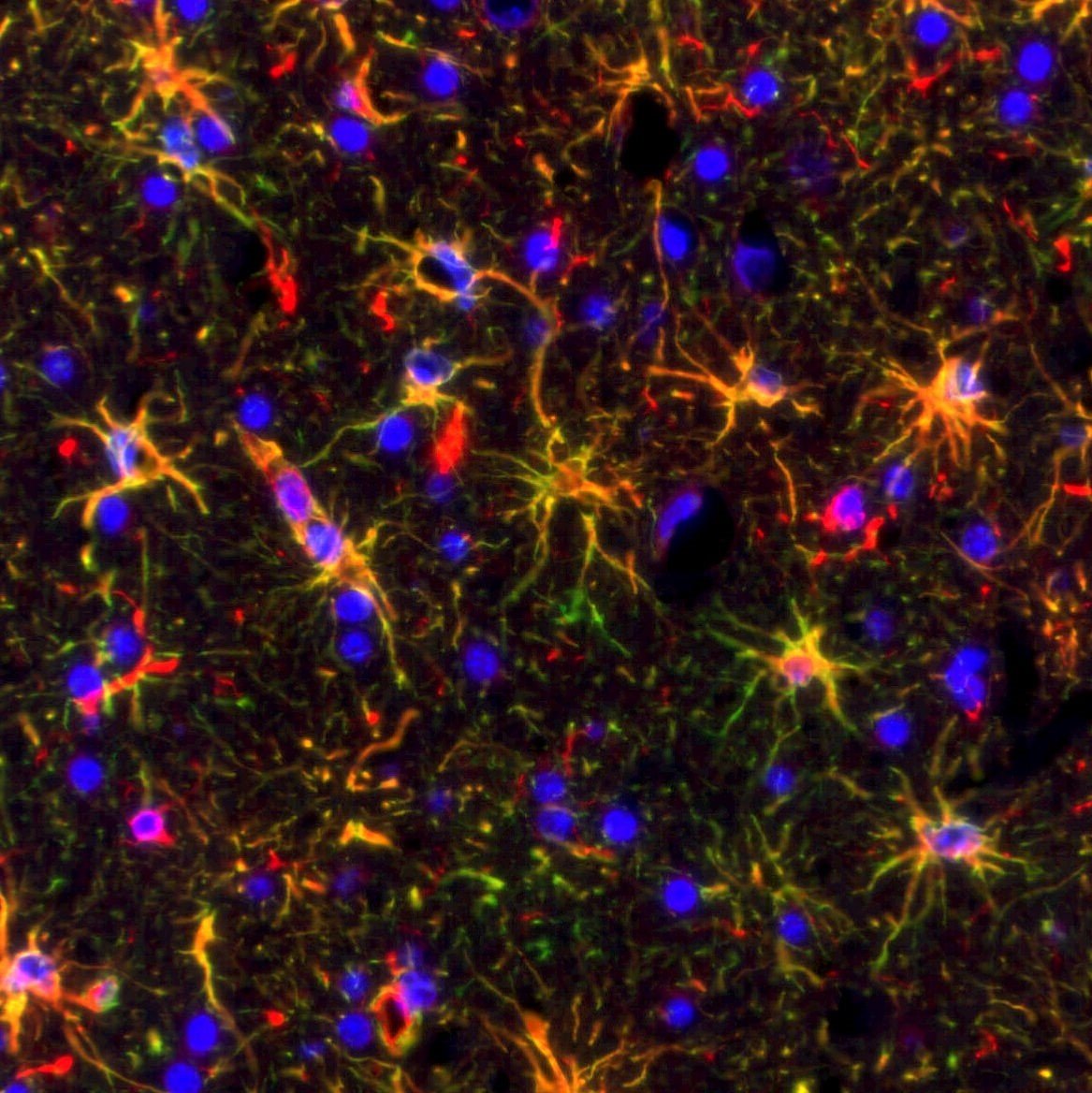
Below are images in which multiplex IF is employed on brain tissues:
 |
 |
|
GFAP (green) and Iba1 (red) with DAPI (blue) counterstain. Note that there are GFAP and Iba1 antibodies stain 2 different cell populations, thus there is no co-localization |
GFAP (green) and Vimentin (red) with DAPI (blue) counterstain. Note that the GFAP and Vimentin antibodies both stain the same cell population and thus the staining is co-localized and the cells appear yellow |
There are some disadvantages to using immunofluorescence.
- The fluorescent signal will fade over time and will eventually disappear. For this reason, it is important to document the results with either standard photomicrographs or whole slide scanning.
- Many tissue elements will autofluoresce (they will fluoresce whether or not they have been stained with IF techniques). Some common autofluorescent elements are: collagen, elastin, lipofuscin, red blood cells, neutrophils, blood vessels, adipocytes. For this reason, it is very important to run isotype controls or even slides that have not been stained with anything to compare to the test tissue.
- Autofluorescence can be minimized in a number of ways:
- Use of frozen sections instead of FFPE tissues (autofluorescence is increased in FFPE tissues)
- Eliminate the use of green dyes (most elements that autofluoresce are in this channel)
- Use of autofluorescence quenching techniques
- Use of tyramide signal amplification to increase the real signal which will overpower autofluorescence
- Photobleaching can also be a problem. This occurs when the fluorescent signal fades due to prolonged exposure of light. This is less of a problem with the Alexa fluor dyes than with traditional fluorochromes.
- Autofluorescence can be minimized in a number of ways:
HSRL’s highly trained team of scientists can assist with your research goals by providing input regarding which IHC or IF markers will be most effective for your studies. We are proficient with both GLP and non-GLP IHC method development and will optimize the antibodies that fit your research needs. Our in-house Hamamatsu S60 slide scanner is capable of scanning with both brightfield and fluorescence. We are happy to consult with you regarding recommendations for tissue collection and fixation procedures that will lead to the best possible staining results.
To request a consult, contact us to start the process. We look forward to hearing from you!