StageBio Distinguished Pathologist Dr. Serge Rousselle, DVM, DACVP, recently gave a webinar presentation on the key considerations of accurate evaluation for medical device safety, efficacy, healing, and biocompatibility in nonclinical assessments.
During this webinar, Dr. Rousselle detailed the important endpoints—aside from the primary objective of safety—essential to compiling a complete histopathology report. The endpoints he covered at length are listed below.
[H2] Critical endpoints for medical device evaluation:
- Biocompatibility
- Healing
- Integration
- Efficacy
- Absorption
- Drug delivery performance, pharmacology, and toxicity (when applicable)
- Downstream safety
You can learn more about why these endpoints are crucial considerations for medical device evaluation here.
Screening methods for medical device evaluation
Dr. Rousselle shared examples of modalities that can be used in conjunction with histopathology to evaluate a medical device. For example, as he explained, key information can be gleaned from imaging modalities such as a high-resolution radiography or an MicroCT.
Other useful modalities pointed out by Dr. Rousselle include:
Macroscopic assessment
A macroscopic assessment can be one of the most important endpoints for certain devices, especially ones that are complex in construction and architecture.
Sampling and preparation strategy
According to Dr. Rousselle, this is a use modality because, as mentioned above, implants are complex in terms of structure and function. They often have multiple components with multiple types of materials. These materials must be sampled to properly assess interactions between any given material and the body. Functional components of a device should be assessed as well.
Quantitative analysis
Increasingly par for the course for many studies, a quantitative analysis can be useful in medical device evaluations that require efficacy metrics.
Ancillary investigative techniques on an as-needed basis
Some implants—such as those for orthopedic, vascular, or neurological applications—can shed particles. Ancillary investigative tools, including scanning electron microscopes and FTIR devices, which can be essential in finding out where micro particulates come from and whether they originated from the implant itself, the delivery procedure or material, or another source.
Histopathology scenario: Absorbable implants
Because they span a wide range of implant types and organ systems, Dr. Rousselle chose absorbable implants as the area of focus for the presentation to highlight the challenges of medical device evaluation.
For example, there are varying modalities of absorption depending on the device, its biomaterial, and how it interacts with the body. Modalities include biological erosion, hydrolytic or enzymatic degradation, phagocytosis, and/or integration. And to call attention to the complexities these absorption modalities can pose in clinical assessment, Dr Rousselle focused on hydrolysis and phagocytosis.
Biocompatibility of absorbable implants: Inflammation vs. Bioabsorption
Once an implant is inserted into the body, there is an initial absorption of serum and blood proteins—and the body may choose to respond in a few different ways:
M2 response (non-inflammatory)
This is the desired “pro-healing” response. Macrophages respond to the site of the implant for what Dr Rousselle referred to as a “clean-up” job of the area. To visualize this, he shared an example of a PLGA absorbable IVC filter.
M1 response (inflammatory)
Conversely, the body can decide to trigger an M1 response, which leads to inflammation that is ultimately observed during the evaluation of the specific medical device and scored accordingly. Illustrating this, Dr. Rousselle shared slides of a collagenous matrix that triggered an M1 response.
![A collagenous matrix provokes an inflammatory response]](https://www.stagebio.com/hs-fs/hubfs/Picture2.png?width=422&height=299&name=Picture2.png)
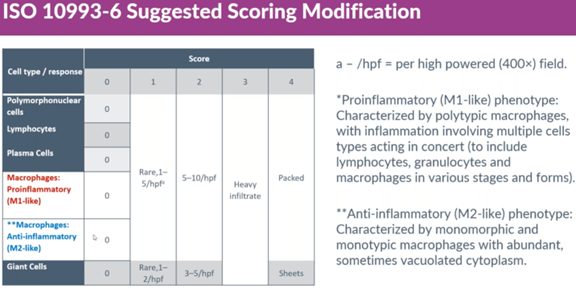
An issue with current ISO 10993-6 irritancy scoring
After discussing the M1 and M2 responses that can occur, Dr. Rousselle also pointed out how modern absorbable implants and materials can provoke an M2 (pro-healing) response with minor inflammation (M1). If these beneficial implants are scored according to ISO 10993-6, then the histopathology review may result in a false positive reactivity score.
![An absorbable stent that provokes minor inflammation but primarily promotes healing]](https://www.stagebio.com/hs-fs/hubfs/Picture3.png?width=422&height=321&name=Picture3.png)
ISO 10993-6 irritancy scoring

A suggested scoring modification
To account for a minor M1 response that can occur from an implant that primarily triggers an M2 response, Dr. Rousselle shared his own scoring method that splits the scoring of macrophages into M1-like and M2-like responses, letting the M1-like response define the reactivity score while the M2-like, non-inflammatory response is excluded from the reactivity computation.
Other challenges of medical device evaluation
While Dr. Rousselle emphasized issues around biocompatibility assessment of absorbable implants, he also covered challenges that can occur throughout the evaluation process, including during tissue collection, and provided possible solutions for each one. Additionally, he detailed and offered solutions to overcome certain scenarios that can occur in histopathology, such as:
- Histology reagents dissolving biomaterial
- Paraffin embedding being too soft
- An implant becoming lost through floatation during microtomy
You can see his proposed solutions for these and other challenges by watching the webinar, now available on demand.
Questions from attendees regarding medical device evaluation best practices
Following the webinar, Dr. Rousselle took questions from attendees. The webinar generated many questions that were addressed with thoughtful discussion.
Here are two of the questions asked during the Q&A, along with Dr. Rousselle’s response:
Q: For absorbable devices, how far into the surrounding tissues do you look for impacts of degradation? Do you typically evaluate the surrounding lymph nodes?
Response: The draining lymph nodes would be a minimum. However, if it’s an implant that’s in a cavity or vascular system, you should also look for derivatives or components into downstream tissues.
Q: What advice would you have for explanting techniques that will maximize your ability to evaluate an implant site, especially with absorbable materials (besides using markers where possible)
Response: If you’re unable to rely on imaging, lacking fiduciary markers, and dealing with an implant site that is not visible, the best and only real solution is to collect samples widely. Beforehand, you would ideally collaborate with those who performed the implantation to a) decide how widely and intensively to sample and b) exert due diligence by producing more slides. This will help ensure you collect the tissue where the implant was.
Conclusion
To take a deeper dive into these topics and learn how to improve histopathological evaluations of medical devices, you can watch Dr. Rousselle’s full webinar here.
