By Dr. Stephanie Shrader, DVM, PhD, DACVP
Director, General Toxicologic Pathology
I recently gave a webinar presentation on important technical considerations and pathology reporting expectations in ocular toxicity studies. My goal was to pass along the knowledge and insight necessary to help the pathologists, study directors, toxicologists, scientists, and everyone else in attendance do the following:
- Describe the ocular anatomic differences between species commonly used for ocular toxicity studies
- Understand why those differences in species are important when setting up or evaluating an ocular toxicity study
- Know the current recommendations for ocular sampling and sectioning techniques
- Summarize the expected components of an ocular toxicity pathology report
Keep reading for a brief recap of my presentation’s takeaways. You can also see the presentation in full here.
Why understanding ocular toxicity is important
According to the World Health Organization, there are more than one billion people globally with some form of vision impairment. With access to the right therapeutic, many of the impairments these people face could be addressed or even prevented.
Leading causes of vision impairment
- Uncorrected refractive errors
- Cataract
- Age-related macular degeneration (AMD)
- Glaucoma
- Diabetic retinopathy
- Corneal opacification
- Trachoma
Through nonclinical ocular toxicity studies, we can develop the ocular drugs and devices that combat and possibly even prevent or cure many of these conditions.
Common ocular administration routes in ocular toxicity studies
The following are some of the most common administration routes used in ocular toxicity studies. Note that potential complications could arise with each route. I discuss these complications in detail in the webinar.
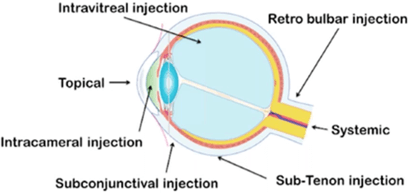 Routes of ocular administration. Pharmaceuticals 2018, 10, 10; doi: 10.3390/pharmaceutics10010010
Routes of ocular administration. Pharmaceuticals 2018, 10, 10; doi: 10.3390/pharmaceutics10010010
Intravitreal
Intravitreal administration is used to treat various vitreoretinal conditions, including:
- AMD
- Diabetic retinopathy
- Retinal vein occlusion
- Endophthalmitis
- Retinitis
Various types of medications are delivered using intravitreal administration. These could be solutions, gels, implants, etc.
Topical
Like intravitreal, topical administration is another commonly used route in both regular practice and toxicity studies. Therapies that treat ocular diseases related to the anterior segment of the eye are often administered topically.
Subretinal
The subretinal region of the eye is the space between the retinal pigmented epithelium (RPE) and the photoreceptors. Many therapeutics delivered using subretinal administration consist of gene and cell therapy for retinal degenerative diseases.
Intracameral
Intracameral isn’t as common as intravitreal or topical administration, but it’s most often used to deliver antibiotics into the anterior chamber. One use case is the prevention of post-surgical endophthalmitis. Intracameral administration lacks a standardized approach or best practices.
Periocular
Periocular administration takes advantage of the permeability of the sclera to deliver retinal drugs and includes subconjunctival, sub-tenant, retrobulbar, peribulbar, and posterior juxtascleral routes. It’s particularly useful for administering sustained-release drugs and drugs such as corticosteroids and ocular anesthetics for surgical procedures.
Study types and drug delivery methods
Ocular toxicity studies are used for a wide range of study types and drug delivery methods:
- Systemic administrative studies (to evaluate on-target and off-target effects in the eye)
- Medical device studies
- Contact lenses
- Intraocular lenses (IOLs)
- Non-pharmacologically active topical and inject substances
- Intracorneal implants and keratoprostheses
- Scleral buckles
- Glaucoma drainage devices
- Resorbable/non-resorbable intravitreal implants
- Retinal prostheses
- Novel surgical techniques
Ocular anatomic differences between commonly used species
Naturally, there are ocular anatomic differences across humans and other species that must be accounted for when setting up and conducting a nonclinical ocular toxicity study. For example, laboratory beagles – like all dogs – have a tapetum in their eye. This structure provides light-sensitive retinal cells with a second opportunity for photo-receptor stimulation, which in turn enhances visual sensitivity at low-light levels. There is no human equivalent.
The key takeaway is that a potential lab animal’s ocular biology must be considered within the context of a toxicity study’s methods and goals. If humans lack an equivalent structure in the eye, the pathologists must decide if that difference is biologically relevant.
I provide more in-depth examples of structural atomic differences between lab animal species and humans, and what those differences mean for ocular toxicity studies, in my presentation.
Necropsy considerations in ocular toxicity studies
The point I drive home regarding necropsy is this: Be gentle. Eyes are typically enucleated at necropsy and fixed whole to minimize artifacts. Additionally:
- Make sure that the eyes (left and right) and adnexa can be differentiated
- Mark the globe with either suture or tissue ink at the 12-o-clock position (this allows for orientation during trimming)
- Use Davidson’s Solution or modified Davidson’s solution for no longer than 24 to 48 hours
- Depending on your study’s endpoint, discuss the best way to handle ocular tissue with your team
Watch my presentation for more ocular toxicity insights
To learn more about each of the topics previewed above, watch the on-demand presentation here. You’ll also learn about:
- Current recommendations for ocular sampling and sectioning
- Proper data collection for ocular toxicity studies
- Critical report components for GLP-compliant general toxicity studies
- How to avoid ocular study issues
- StageBio’s ocular capabilities
About StageBio
StageBio is a leading provider of GLP-compliant necropsy, histology, pathology, and specimen archiving and biorepository services for the biopharmaceutical, medical device, academic, and contract research industries. The company operates four GLP laboratories as well as three GLP specimen archiving facilities in the U.S., along with one GLP laboratory in Europe. StageBio will continue to make investments in facility and technology infrastructure to meet the growing demand for high-quality histopathology services globally. StageBio has a team of 30+ board-certified veterinary pathologists and more than 100 laboratory technicians on staff supporting our unified commitment to quality, scientific integrity, and client satisfaction. Learn more at stagebio.com.
