Peripheral arterial disease (PAD) affects more than 200 million people worldwide, with its most severe manifestation, chronic limb-threatening ischemia, potentially leading to imminent limb loss in up to 11% of patients. While endovascular methods, such as drug-eluting stents, have improved acute outcomes, long-term restenosis rates remain high, especially in below-the-knee (BTK) arteries. Permanent metallic stents pose additional challenges, including impaired vasomotor tone, chronic inflammation, and stent fractures, which can complicate future bypass surgeries. These limitations underscore the need for innovative solutions, such as drug-eluting resorbable scaffolds.
StageBio Distinguished Pathologist Dr. Serge D. Rousselle co-authored a 2025 study to assess the viability of bioresorbable scaffolds as alternatives to permanent metallic stents and first-generation poly-L-lactic acid (PLLA) scaffolds.
To conduct this preclinical study, Dr. Rousselle and his fellow researchers evaluated the safety, biocompatibility, and performance of these bioresorbable scaffolds in small-caliber porcine peripheral arteries. The study’s findings suggest that these bioresorbable scaffolds are safe and effective in treating BTK lesions in peripheral arteries.
Evaluating MOTIV bioresorbable scaffolds
The MOTIV scaffold, developed by REVA Medical, is a sirolimus-eluting bioresorbable scaffold made from Tyrocore. A novel polycarbonate polymer, Tyrocore, features higher tensile strength and ductility compared to traditional PLLA scaffolds, along with radiopacity equivalent to that of metallic stents. The scaffold elutes sirolimus within 30 days and fully resorbs within four years, leaving the vessel uncaged.
Study details
Twenty MOTIV scaffolds of varying sizes were implanted in the internal iliac arteries of nine Yorkshire swine.
The study included two groups:
- Group 1 (30-day follow-up)
- Group 2 (90-day follow-up)
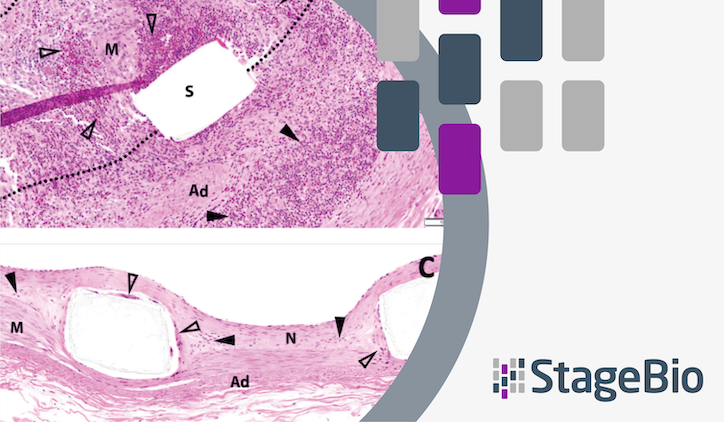
Optical coherence tomography, histopathology, and scanning electron microscopy were used to assess vascular healing, scaffold integrity, and biocompatibility. Parameters such as neointimal thickness, percent area stenosis, strut coverage, and inflammation were analyzed.
Findings and Results
The study demonstrated promising results:
- Vascular Healing: All vessels remained patent at both 30 and 90 days, with complete reendothelialization and mature neointima formation. Neointimal thickness averaged 0.22 mm at 30 days and 0.18 mm at 90 days, with percent area stenosis of 28% and 24%, respectively.
- Scaffold Integrity: No malapposition, stent fractures, or late strut discontinuities were observed. The scaffolds retained their structural integrity and radial strength throughout the study.
- Biocompatibility: Inflammation scores were low, with minimal lymphocyte, macrophage, and multinucleated giant cell infiltration. One animal exhibited granulomatous inflammation, likely due to individual variability in immune response.
- SEM Analysis: Complete endothelial coverage was observed, with no thrombosis, fibrin deposition, or platelet adhesion. Minor surface artifacts were attributed to preparation processes.
Advancing PAD treatment with a bioresorbable scaffold constructed from a novel polycarbonate polymer
The MOTIV bioresorbable scaffold demonstrated excellent safety, biocompatibility, and performance in preclinical settings, addressing the limitations of permanent metallic stents and first-generation PLLA scaffolds. These findings suggest that MOTIV scaffolds could offer a viable alternative for treating BTK lesions, providing a solution to the challenges posed by PAD. While preclinical results must be interpreted cautiously due to the absence of atherosclerotic disease in animal models, the study adds valuable insights into vascular healing and scaffold performance. Combined with positive clinical outcomes, MOTIV scaffolds have the potential to expand the interventional toolkit for PAD, improving long-term outcomes and patient quality of life.
Access the paper published in Scientific Reports
Download the paper here for full details on the study and its findings regarding the effectiveness, biocompatibility, and safety of drug-eluting resorbable scaffolds.