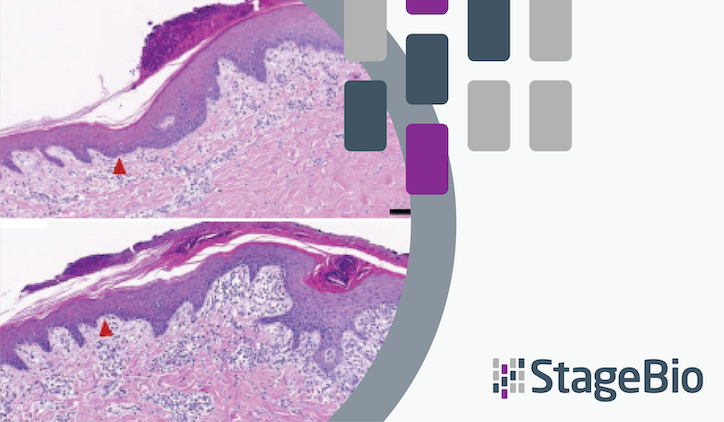
To understand how various rates of water evaporation impact wound healing, StageBio director of digital and quantitative pathology Dr. Thomas Lemarchand and Image Analyst Fabian Kukla co-authored a study to test in vitro and in vivo water evaporation rates from the cellulose dressing epicitehydro when combined with different secondary dressings. For that purpose, the researchers evaluated wound healing efficacy in a porcine donor site model using quantitative pathology including Artificial intelligence and image analysis.
By the end of the study titled “Orchestrating the Dermal/Epidermal Tissue Ratio during Wound Healing by Controlling the Moisture Content,” Dr. Lemarchand and his fellow researchers including Fabian Kukla found:
- High-moisture content dressing can have a varying impact on different healing processes, depending on the secondary wound dressing it’s used with
- Combining a high-moisture content dressing with a nonocclusive dressing may support re-epithelialization
- The combination of a high-moisture content dressing with an occlusive secondary would dressing resulted in significant regeneration of granulation/new dermal tissue
The observation that high water evaporation rates obtained with a proper secondary dressing (e.g., cotton gauze) promotes improved and faster re-epithelization , whereas low water evaporation from an occlusive secondary dressing is boosting the regeneration of new and intact dermal tissue can help inform future innovations in clinical wound management.
To learn about the study in greater detail and evaluate its findings, you can download and read “Orchestrating the Dermal/Epidermal Tissue Ratio during Wound Healing by Controlling the Moisture Content” here.
About StageBio
StageBio is a leading provider of GLP-compliant necropsy, histology, pathology, and specimen archiving and biorepository services for the biopharmaceutical, medical device, academic, and contract research industries. The company operates four GLP laboratories as well as three GLP specimen archiving facilities in the U.S., along with one GLP laboratory in Europe. StageBio will continue to make investments in facility and technology infrastructure to meet the growing demand for high-quality histopathology services globally. StageBio has a team of 30+ board-certified veterinary pathologists and more than 100 laboratory technicians on staff supporting our unified commitment to quality, scientific integrity, and client satisfaction. Learn more at stagebio.com.