Intravitreal drug development is challenging, primarily due to the sensitivity of the eye and the limited safe injection volume. One critical factor of intravitreal tolerability and safety is the pH level of a given drug formulation. While pH levels between 5.5 and 7.4 are generally well tolerated, acidic solutions below this range raise concerns about potential adverse effects.
StageBio and Altasciences partnered to evaluate the ocular tolerability of formulations with pH levels of 4.0, 5.0, and 7.0 in New Zealand (NZW) and Dutch Belted (DB) rabbits over 57 days. The resulting study and its findings were published in a poster co-authored by StageBio Senior Pathologist Dr. Quinci Plumlee, DVM, MS, DACVP.
The poster, titled “Evaluation of Formulation pH Tolerability in New Zealand White and Dutch Belted Rabbits Post Intravitreal Administration,” would go on to win the Best Ocular Poster Award at the 64th Annual Society of Toxicology (SOT) Meeting and ToxExpo.
You can access the poster by contacting us here or continue reading for a summary of the study and its key findings.
Study Overview: Intravitreal Drug Formulation Tolerability
The study aimed to evaluate the tolerability of formulations with pH levels of 4.0, 5.0, and 7.0 following a single intravitreal injection in both eyes of each rabbit. The research team divided 18 rabbits (6 males and 3 females of each breed) into three groups:
- Group 1 (Vehicle Control): 150 mM Sodium Chloride, pH 7.0
- Group 2: 20 mM Acetate, 150 mM Sodium Chloride, pH 4.0
- Group 3: 20 mM Acetate, 150 mM Sodium Chloride, pH 5.0
Each rabbit received a single bilateral intravitreal injection of 100 µL, followed by an 8-week observation period. The study employed a comprehensive set of ocular assessments, including weekly:
- Draize scoring
- Intraocular pressure (IOP) measurements
- Ophthalmic examinations
- Fundus imaging
- Electroretinography (ERG)
- Confocal Scanning Laser Ophthalmoscopy/Optical Coherence Tomography (cSLO/OCT) imaging.
At the end of the study, StageBio conducted histological evaluations to assess retinal morphology.
Key Findings
Group 1 (Vehicle Control, pH 7.0)
No adverse effects were observed in the vehicle control group. Ophthalmologic evaluations, fundus imaging, and OCT scans revealed no evidence of retinal degeneration or atrophy.
Group 2 (Acidic Solution, pH 4.0)
The acidic solution at pH 4.0 demonstrated significant intolerance, with focal retinal degeneration observed in 7 of 12 eyes by Day 12 of the study. By Day 57, retinal degeneration/atrophy was confirmed in these eyes, accompanied by additional findings such as:
- Minimal hypertrophied retinal pigment epithelium (RPE)
- Minimal vitreal mononuclear cell infiltrates
- Rare pigmented cells (macrophages and/or RPE) within the retina (DB rabbits only)
Histological evaluations revealed loss of distinct retinal layers, hypertrophy of the RPE, and pigmented cells within the affected retina. These findings raise concerns about the safety of intravitreal formulations with a pH of 4.0.
Group 3 (Acidic Solution, pH 5.0)
The pH 5.0 formulation showed better tolerability compared to pH 4.0. No evidence of retinal degeneration or atrophy was observed. Additionally, findings were limited to procedure-related effects, such as injection tracks and minimal choroidal mononuclear cell infiltrates.
These results suggest that formulations with a pH closer to physiological levels may be safer for intravitreal use.
Electroretinography (ERG) Analysis
ERG analysis provided insights into the functional impact of acidic solutions on photoreceptor activity:
- Dutch Belted Rabbits: Mild decreases in scotopic -24dB responses were observed in Groups 2 and 3 at Weeks 4 and 8. Group 2 also showed mild decreases in Scotopic 0dB a-wave responses at Weeks 4 and 8, while Group 3 exhibited similar changes at Week 8. No changes were noted in scotopic 0dB b-wave or photopic flicker responses.
- New Zealand White Rabbits: No differences in scotopic or photopic responses were observed at any time point, indicating breed-specific variations in response to acidic solutions.
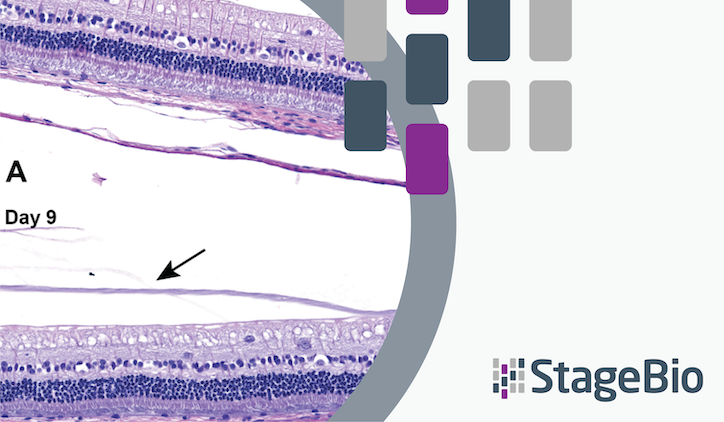
Representative Imaging and Histology
The study utilized advanced imaging techniques to document retinal abnormalities. Fundus images, IR cSLO images, and OCT scans revealed round, discolored lesions and retinal degradation in Group 2 animals. Histological evaluations confirmed these findings, highlighting focal retinal degeneration, hypertrophy of the RPE, and pigmented cells within the affected retina.
Implications for Intravitreal Drug Development
The findings of this study underscore the importance of pH in intravitreal drug formulations. Acidic solutions at pH 4.0 were associated with significant retinal degeneration, raising concerns about their safety for clinical use. In contrast, formulations at pH 5.0 showed better tolerability, with no evidence of retinal damage. These results suggest that pH ranges closer to physiological levels should be prioritized in intravitreal drug development.
Additionally, the study highlights the need for breed-specific considerations, as DB rabbits exhibited more pronounced functional changes compared to NZW rabbits. This variability underscores the importance of using diverse animal models to assess the safety and efficacy of intravitreal formulations.
Direction for Future Intravitreal Tolerability Studies
While this study provides valuable insights into the tolerability of acidic intravitreal formulations, further research is needed to assess their long-term effects on retinal morphology and physiology. Key areas for future investigation include:
- Evaluating the impact of repeated injections of acidic solutions
- Exploring the mechanisms underlying retinal degeneration caused by low pH
- Assessing the safety of formulations with intermediate pH levels (e.g., 5.5–6.5)
- Investigating breed-specific differences in response to intravitreal formulations
Access the full ocular poster for more detailed findings
Intravitreal drug development requires careful consideration of formulation pH to ensure safety and efficacy. This study demonstrates that acidic solutions at pH 4.0 can lead to retinal degeneration, while formulations at pH 5.0 are better tolerated. These findings provide critical guidance for the development of intravitreal drugs, emphasizing the need to prioritize pH ranges closer to physiological levels. As the field continues to evolve, further research will be essential to refine our understanding of pH tolerability and optimize intravitreal drug formulations.
By advancing our knowledge of pH tolerability, we can pave the way for safer and more effective treatments for ocular diseases, ultimately improving patient outcomes and quality of life.
Contact us to access the full poster for a more in-depth look at the study methods and takeaways, including imagery that visualizes key findings.