The limitations of current PFO occluders
According to the Mayo Clinic, approximately one in four people have a hole between the left and right chambers of the heart known as a patent foramen ovale, or PFO. First-generation PFO occluders were designed to stop any resulting blood flow between the two chambers.
However, these occluders have historically exhibited bulk erosion, dislodgement, and a rigidity that leaves them unable to conform to atrial anatomies. Because many people with a PFO have the condition since the time of birth or young adulthood, these limitations should be addressed to accommodate long life expectancies.
This promising pre-clinical study demonstrated good safety and efficacy of a novel PFO closure device
Dr. David Garlick, a senior pathologist at StageBio, and Michelle Olson MS, a senior scientist at StageBio, co-authored a study published in the Journal of Cardiology on the performance, safety, and biocompatibility of a novel, low-profile, and fully retrievable Encore Medical PFO closure device.
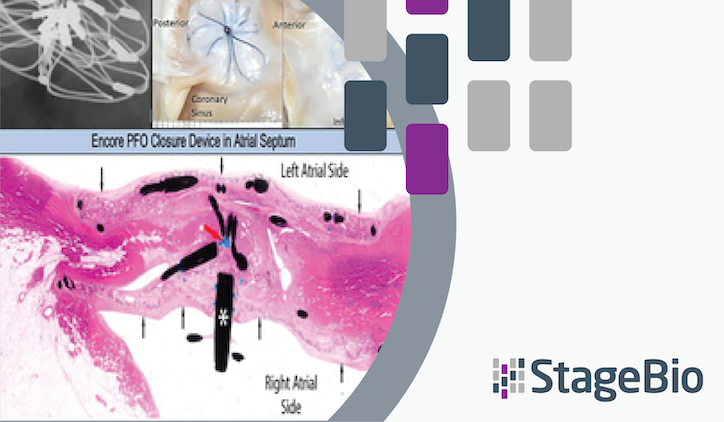
To test the device’s feasibility and safety, six swine received the novel PFO occluder via percutaneous implantation. All six animals survived for the full duration of the study, which was a period of 140 days. After 29 days, transthoracic echocardiography (TTE) showed successful device retention with no blood flow across the septum or thrombus across all specimens.
Gross and histopathologic analyses at termination revealed that the Encore PFO closure device retained implant integrity with complete integration of the devices into the septum as well as complete re-endothelialization of a mature, relatively thin neoendocardium. Additionally, surface fibrin deposition or thrombosis was not observed.
Characteristics of the Encore PFO occluder
- Articulating frame structure that conforms to atrial anatomies
- Favorable device retention and biocompatibility
- A good feasibility and safety profile following 140-day study duration
Learn more about the novel Encore PFO closure device
Access the study, “Performance, safety, and biocompatibility of a novel PFO closure device in a long-term porcine model,” here.