Use Cases and Benefits of Immunohistochemistry, Immunofluorescence, and In Situ Hybridization
In this recent webinar, we took an in-depth look at the benefits and applications of using immunohistochemistry (IHC) and immunofluorescence (IF) molecular assays for pre-clinical and clinical research. We also examined some of the uses and benefits of in situ hybridization (ISH).
If you couldn’t make the webinar, don’t worry. This blog post is the second in a two-part series written to recap how IF, IHC, and ISH can benefit your research efforts. You can find the first blog post in this series, written by fellow presenter Dr. Matthew Buccellato, here. It focuses more on the basics surrounding IHC and IF, along with listing many research tasks where these molecular assays can add immense value. For our second blog post, we’ll pivot to look at some of the many uses for IHC, IF, and ISH.
What are applications of IHC, IF, ISH in clinical and non-clinical studies?
Regarding IHC and IF assays, these are used to detect particular endogenous or exogenous proteins or peptide sequences. While IHC assays help researchers to visualize exactly where proteins are localized within tissues, using an IF assay can help detect and distinguish multiple targets within a given section.
Meanwhile, ISH is a technique that utilizes a single-stranded nucleic acid sequence (a probe) which hybridizes with specific sequences of either RNA or DNA within the tissue sample which can then be visualized using a chromogenic or fluorescent method. This reveals the location of specific nucleic acid sequences to detect the presence or absence of a particular DNA or RNA sequence.
The ability of IHC and IF assays, as well as ISH, to detect and isolate specific targets within tissues has many applications for pathologists, toxicologists, pharmacologists, and others, such as:
Pharmacology Studies
Pre-clinical researchers can use IHC, ISH, or IF to identify, classify, and quantify key biomarkers in pharmacology studies Examples include phenotyping inflammatory cells in animal models of immunopathologic disease and evaluating efficacy in animal models of disease (e.g. IHC for islet, beta, and alpha cell masses in models of diabetes). This provides a mean by which response to treatment can be monitored.
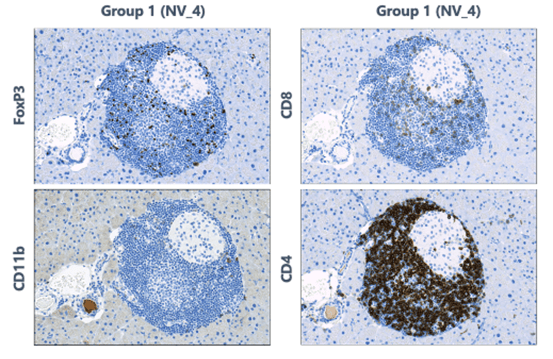
An example of a pharmacological application of IHC
This example shows immune mediated insulitis in the pancreases of mice. Group 1 is the control for this particular study. Biomarkers of immune inflammation are expressed. The size of the islets and the subset of cells indicate the development of diabetes.
Tissue target distribution studies and tissue cross reactivity (TCR) studies
IHC assays provide the ability to map the presence of targets across many tissues with commercially available antibodies for tissue target distribution studies. IHC assays also allow for the use of tissues or matrices of small, extracted cores (tissue microarrays, or TMAs).
In addition, IHC assays for potential therapeutic monoclonal antibodies can be used to identify target and off-target binding of antibodies in human tissue.
Biodistribution in non-clinical disease models
Here’s a specific example of biodistribution.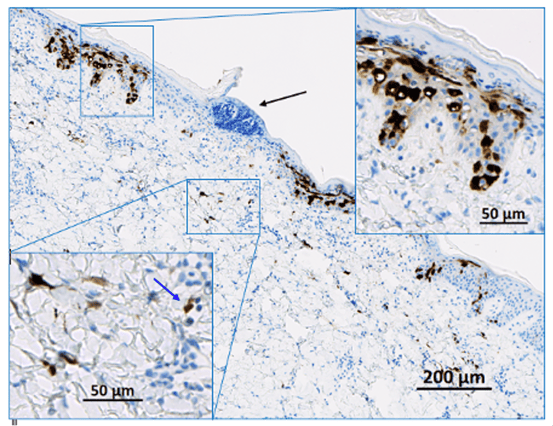
The image shows the injection site of a novel gene therapeutic product in pig skin. A reporter molecule is expressed, which is then detected with an anti-reporter molecule antibody.
As demonstrated here, when injected locally, a reporter gene can distribute in normal or diseased skin. Using an indirect imaging approach, you can see some cells in the epidermis and upper right inset and the dermis and the lower left inset are stained. The cellular distribution and the intensity of the expression of the reporter molecule are observable, which allows for the potential to study, predict, and correct skin diseases.
Biodistribution of native RNA at the cellular level
ISH reveals the native RNA, DNA, or modified stabilized and/or capped therapeutic nucleic acids at the cellular level. And while you can’t use long probes due to the resulting fixation breaking down nucleic acids, innovations in technology, such as the ACDbio platform, let researchers detect RNA down to a single copy.
An example of ISH
The below image shows mRNA expression of a gene therapy product in the brain of a mouse model of disease.
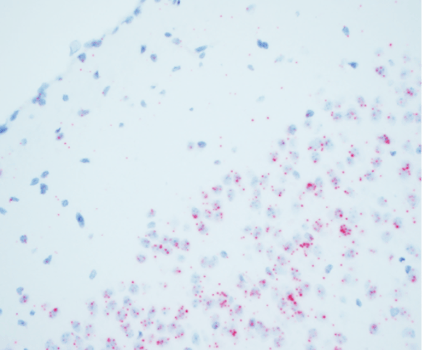
Clinical studies for immuno-oncology
IHC assays can be used to identify and classify inflammatory markers such as CD3 and other T-cell subsets, as well as macrophages, neutrophils, and others. Researchers can also use IHC assays to identify specific tumor markers such as PD1, PDL1, and EGFR—or the combinations of markers within the same sections (multiplexing).
Oncology
IHC and IF assays have many useful applications in oncology—from phenotyping inflammatory infiltrates (as discussed above) to determining the lineage of neoplastic cells that are identified in routine Hematoxylin and Eosin (H&E) stains. For example, cytokeratins can be used to identify tumors of epithelial origin, vimentin can be used to identify tumors of mesenchymal origin, and CD45 can be used to identify neoplasm cells of lymphoid origin. Lymphoid tumors can be further characterized using CD3 for T-cells and CD19 or CD20 for B-cells.
Additionally:
- Other markers can be used to characterize tumors:
- Markers, such as Ki67 or PCNA can be used to determine how rapidly neoplasm cells are proliferating
- IHC can also be used to distinguish apoptosis from necrosis by using markers such as cleaved caspase 3 for apoptosis
- Endothelial cell markers, such as CD31, can be used to evaluate tumor angiogenesis
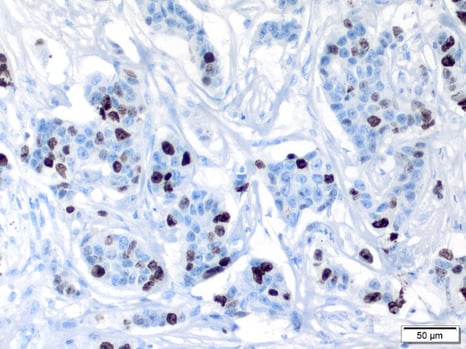
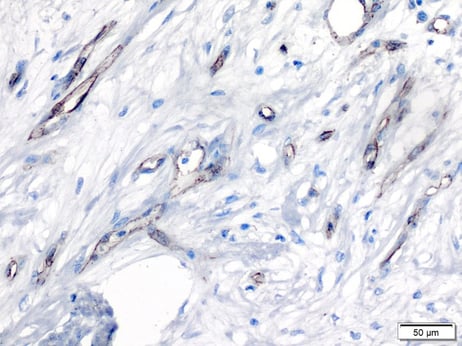
Example of markers used to determine proliferation
The Ki67 marker in human breast carcinoma:
The CD31 marker in human breast carcinoma:
Identification of human cells in animal tissues
Researchers can use the IHC and IF or ISH to identify human cells in animal tissues, which is particularly useful for biodistribution, biotoxicity, and tumorigenicity studies using cell-based therapies.
Commonly used markers include:
- Human mitochondria (hMito)
- Human nuclear antigen
- STEM121
- STEM101
- alu ISH
Characterizations of animal models of disease
IHC can also be used to characterize animal models of disease. This is helpful in understanding the pathogenesis of disease, and it can be useful when evaluating efficacy or toxicity in your animal model. For example, characterizing inflammatory infiltrates in models of an autoimmune disease or characterizing neuro-degenerative diseases, such as Alzheimer’s.
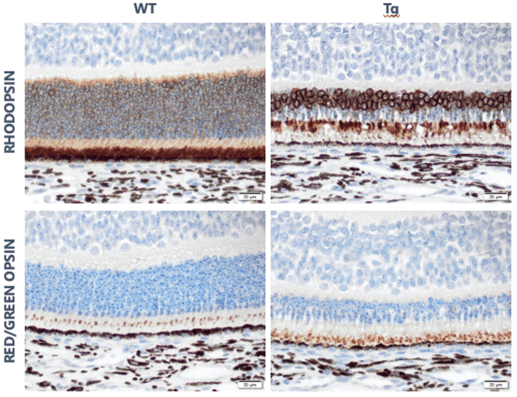
Example of animal model with disease characterization
Below are slides that show the pig model of retinal degeneration. Note the loss of rods (rhodopsin) with the sparing of cones (red/green opsin).
What are the benefits of outsourcing your IHC, IF, and ISH studies?
When you outsource your IHC, IF, and ISH studies to StageBio, you leverage our years of experience, state-of-the-art staining platforms, and cost and time efficiencies. With early engagement, we can help you design your studies and select the best markers and methods. StageBio maintains high-quality, high-throughput slide production for our clients, and we also offer services for:
- Histology (embedding, sectioning; both FFPE and OCT-embedded frozen)
- IHC marker optimization
- Pathologist validation of markers
- IHC/IF/ISH slide staining
- Whole slide imaging
- Digital image analysis
- Pathology evaluation and reporting
Conclusion
To learn more about the benefits of IHC, IF, and ISH, go here. Or, if you’re ready to talk about outsourcing your IHC/IF research and how StageBio can support you, go here.